Jump to Introduction & Chronology
Jump back to Previous: Uncle Tungsten - IX. Volta to Maxwell to Hertz
Uncle Tungsten
p181 Schubert lieder, “Nachtgesang”, not “The Linden Tree”, (referenced in The Magic Mountain) but still... Music over the radio and on the phonograph. I've already talked about what a revolution that was, to be able to hear music when you pleased without needing to play it yourself.
p186 [Of his older brother Michael’s descent into psychosis after his boarding school experiences.] I became terrified of him, for him, of the nightmare which was becoming reality for him, the more so as I could recognize similar thoughts and feelings in myself, even though they were hidden, locked up in my own depths. What would happen to Michael, and would something similar happen to me, too? It was at this time that I set up my own lab in the house, and closed the doors, closed my ears, against Michael’s madness. It was at this time that I sought for (and sometimes achieved) an intense concentration, a complete absorption in the worlds of mineralogy and chemistry and physics, in science -- focusing on them, holding myself together in the chaos. It was not that I was indifferent to Michael; I felt a passionate sympathy for him, I half-knew what he was going through, but I had to keep a distance also, create my own world from the neutrality and beauty of nature, so that I would not be swept into the chaos, the madness, the seduction, of his.
Chapter 16 - Mendeleev’s Garden
...
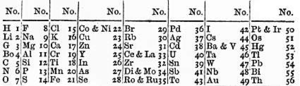
p188 [After first seeing a display -- a large cabinet with cubicles displaying samples -- labeled “The Periodic Classification of the Elements -- after Mendeleeff” at the re-opened Science Museum in South Kensington in 1945] I had, by this time become familiar with the properties of many elements and I knew they formed a number of natural families, such as the alkali metals, the alkaline earth metals, and the halogens. These families ([Dmitri] Mendeleev called them “groups”) formed the verticals of the table, the alkali and alkaline earth metals to the left, the halogens and inert gases to the right, and everything else in four intermediate groups in between. The “groupishness” of these intermediate groups was somewhat less clear -- thus in Group VI, I saw sulfur, selenium, and tellurium, but what was oxygen doing, heading the group? There must be some deeper principle at work -- and indeed there was. This was printed at the top of the table... The deeper principle, I saw, was valency. The term valency was not to be found in my early Victorian books, for it had only been properly developed in the late 1850s, [it's interesting that the term predates the understanding of what was actually happening. You can imagine a "Who's on First" conversation between a chemist from the middle of the 19th century and one from a century later] and Mendeleev was one of the first to seize on it and use it as a basis for classification, to provide what had never been clear before: a rationale, a basis for the fact that elements seemed to form natural families, to have deep chemical and physical analogies with one another. Mendeleev now recognized eight such groups of elements in terms of their valencies.
p189 Thus the elements in Group I, the alkali metals, had a valency of 1: one atom of these would combine with one atom of hydrogen to form compounds such as LiH, NaH, KH, and so on... The elements of Group II, the alkaline earth metals, had a valency of 2, and so would form compounds such as CaCl2, SrCl2, BaCl2, and so on. The elements of Group VIII had a maximum combining power of 8.
...
Every element echoed the properties of the one above, was a slightly heavier member of the same family. The same melody, so to speak, was played in each period [the horizontal lines of the table] -- first an alkali metal, then an alkaline earth metal, then six more elements, each with its own valency or tone -- but played in a different register (it was impossible to avoid thinking of octaves and scales here, for I lived in a musical house, and scales were the periodicity I heard daily).
p190 It was eightness which dominated the periodic table before me...
I got a sudden, overwhelming sense of how startling the periodic table must have seemed to those who first saw it -- chemists profoundly familiar with seven or eight chemical families, but who had never realized the basis of these families (valency), nor how all of them might be brought together into a single overarching scheme. I wondered if they had reacted as I did to this first revelation: “Of course! How obvious! Why didn’t I think of it myself?”
...I had the sense that it harbored a marvelous secret, but it was a cryptogram without a key -- why was this relationship so?
...To have perceived an overall organization, a superarching principle uniting and relating all the elements, had a quality of the miraculous, of genius. And this gave me, for the first time, a sense of the transcendent power of the human mind, and the fact that it might be equipped to discover or decipher the deepest secrets of nature, to read the mind of God.
I'm psychic!
...
Unless I had a blackout and missed it, he has not explained what valency means. And I’m not sure, before I hit Wiki, if Mendeleev was basing this on the combinations the elements were known to make, or if he understood about the distribution of electrons in shells. No, he didn't understand what was actually happening.
p191 [His second visit the following day] ...Metals had long been recognized as a special category of elements, and now one could see, in a single synoptic glance, how they occupied three-quarters of the realm -- all of the west side, most of the south -- leaving only a smallish area, mostly in the northeast, for the nonmetals. A jagged line, like Hadrian’s Wall, separated the metals from the rest, with a few “semimetals,” metalloids -- arsenic, selenium -- straddling the wall. One could see the gradients of acid and base, how the oxides of the “western” elements reacted with water to form alkalis, the oxides of the “easter” elements, mostly nonmetals, to form acids. One could see, again at a glance, how the elements on either border of the realm -- the alkali metals and halogens, like sodium and chlorine, for example -- showed the greatest avidity for each other and combined with explosive force, forming crystalline salts with high melting points which dissolved to form electrolytes; while those in the middle formed very different compounds -- volatile liquids or gases which resisted electric currents. One could see, remembering how Volta and Davy and Berzelius ranked the elements into an electrical series, how the most strongly electropositive elements were all to the left, the most strongly electronegative to the right. Thus it was not just the placement of the individual elements, but trends of every sort that hit the eye when one looked at the table.
Seeing the table, “getting” it, altered my life. I took to visiting it as often as I could. I copied it into my exercise book and carried it everywhere; I got to know it so well -- visually and conceptually -- that I could mentally trace its paths in every direction... It was like a garden, the garden of numbers I had loved as a child -- but unlike this, it was real, a key to the universe. I spent hours now, enchanted, totally absorbed, wandering, making discoveries, in the enchanted garden of Mendeleev.
I like the way Sacks gave us an insight into his state of mind, and concern about that, in the preceding chapter. Also, I have to say that this is more of what I would have expected from a book titled "The Periodic Table." I wonder if Sacks read that, was disappointed, and eventually wrote this to fulfill the promise of Primo Levi's title?
...
His [Mendeleev’s] book, his life, did not disappoint me. He was a man of encyclopedic interests. He was also a music lover and a close friend of Borodin (who was also a chemist). And he was the author of the most delightful and vivid chemistry text ever published, The Principles of Chemistry.
...
p196 It was nearly twenty years from Mendeleev’s first interest in classification to the emergence of his periodic table in 1869. This long pondering and incubation (so similar, in a way, to Darwin’s before he published On the Origin of Species) was perhaps the reason why, when Mendeleev finally published his Principles, he could bring a vastness of knowledge and insight far beyond any of his contemporaries -- some of them also had a clear vision of periodicity, but none of them could marshal the overwhelming detail he could.
...
...Mendeleev had gone to Karlsruhe with Borodin [the conference in 1860 when the atomic weights of the elements had finally been sorted out] (this was a musical as well as a chemical journey, for they stopped at many churches en route, trying out the local organs for themselves)...
*Snort!*
...
p198 Moving between conscious calculation and hunch, between intuition and analysis, Mendeleev arrived within a few weeks at a tabulation of thirty-odd elements in order of ascending atomic weight, a tabulation that now suggested there was a recapitulation of properties with every eighth element. And on the night of February 16, 1869, it is said, he had a dream in which he saw almost all of the known elements arrayed in a grand table. The following morning, he committed this to paper.
This is here mostly because of the similarity to Adrian’s composing process toward the end of Doctor Faustus, but then there is the footnote...
(Note: ...if one looks at the actual table Mendeleev sketched, one can see that it is full of transpositions, crossings-out, and calculations in the margins. It shows, in the most graphic way, the creative struggle for understanding which was going on in his mind. Mendeleev did not wake from his dream with all the answers in place, but, more interestingly, perhaps, woke with a sense of revelation, so that within hours he was able to solve many of the questions that had occupied him for years.)
p199 The logic and pattern of Mendeleev’s table were so clear that certain anomalies stood out at once. Certain elements seemed to be in the wrong places, while certain places had no elements. On the basis of his enormous chemical knowledge, he repositioned half a dozen elements, in defiance of their accepted valency and atomic weights. In doing this, he displayed an audacity that shocked some of his contemporaries (Lothar Meyer, for one, felt it was monstrous to change atomic weights simply because they did not “fit”).
Thanks to what Sacks has told us about Mendeleev’s musical background, his confidence in this is easy to understand. A musician can knows, by looking at a score, what notes are going to “sound” like. It would be surprising if they didn’t sound as they “should.” And a person with a good ear could easily correct a score, after hearing music performed, to match the reality.
I don’t think we’ve had a good tangent in a while -- how different is the experience of a piece of music for someone who reads music and can “experience” the music visually by viewing the score (again, a topic from Doctor Faustus) versus someone who doesn’t read music and only plays by ear. Is the symbolic representation of music a benefit or a detriment to true appreciation? Not being musical in this sense, I haven’t a clue about this.
In an act of supreme confidence, Mendeleev reserved several empty spaces in his table for elements “as yet unknown.” He asserted that by extrapolating from the properties of the elements above and below (and also, to some extent, from those to either side) one might make a confident prediction as to what these unknown elements would be like. He did exactly this in his 1871 table, predicting in great detail a new element (“eka-aluminum”) which would come below aluminum in Group III. Four years later just such an element was found, by French chemist Lecoq de Boisbaudran, and named (either patriotically, or in sly reference to himself, gallus, the cock) gallium.
...There were some initial discrepancies between Lecoq’s observations and Mendeleeve’s predictions [of gallium], but all of these were rapidly resolved in favor of Mendeleev. Indeed, it was said that Mendeleev had a better grasp of the properties of gallium -- an element he had never even seen-- than the man who actually discovered it.
p200 Suddenly Mendeleev was no longer seen as a mere speculator or dreamer, but as a man who had discovered a basic law of nature, and now the periodic table was transformed from a pretty but unproven scheme to an invaluable guide which could allow a vast amount of previously unconnected chemical information to be coordinated...
p201 The keystone to the whole table, curiously, was not anticipated by Mendeleev, and perhaps could not have been, for this was not a question of a missing element, but of an entire family or group. When argon was discovered in 1894 -- an element which did not seem to fit anywhere in the table -- Mendeleev denied at first that it could be an element and thought it was a heavier form of nitrogen (N3, analogous to ozone, O3). But then it became apparent that there was a space for it, right between chlorine and potassium, and indeed, for a whole group coming between the halogens and the alkali metals in every period. This was realized by Lecoq, who went on to predict the atomic weights of the other yet-to-be-discovered gases -- and these, indeed, were discovered in short order. With the discovery of helium, neon, krypton, and xenon, it was clear that these gases formed a perfect periodic group, a group so inert, so modest, so unobtrusive, as to have escaped for a century the chemist’s attentions. The inert gases were identical in their inability to form compounds; they had a valency, it seemed, of zero.
I followed up on a footnote reference to Linus Pauling on p204-5 and found a bunch of good stuff, THIS and in particular THIS. And THIS offers an alternate historical perspective -- and do note that great quote from Newton which, again, seems to prefigure what would come to be known as the strong force. And HERE’s a version of the periodic table that Sacks and I could both love. It maps electronegativity to the elements on the table. The least electronegative elements are on the left while the most electronegative elements are mostly on the upper right near fluorine and chlorine. But, there's also a pool of heightened electronegativity in the middle of the metals -- notice how tungsten stands out. This, I believe, is due to the “metallic” bond where atoms share a bunch of elements instead of actually swapping them. This is also why metals are good conductors of electricity -- something else Sacks keeps talking about but never explains. These pools of shared electrons also are the reason metals are malleable. I just noticed that the other element that stands out in the middle is gold, of course.
p210 While Mendeleev saw the periodic table primarily as a tool for organizing and predicting the properties of the elements, he also felt it embodied a fundamental law, and he wondered on occasion about “the invisible world of chemical atoms.” For the periodic table, it was clear, looked both ways: outward to the manifest properties of the elements, and inward to some as-yet-unknown atomic property which determined these...
After publishing this I ran into something interesting in Wiki that I'm going to quote below:
Continuing Johann Wolfgang Döbereiner's work with triads and Jean-Baptiste Dumas' families of similar elements, [John Newlands] published in 1865 his 'Law of Octaves', which stated that 'any given element will exhibit analogous behaviour to the eighth element following it in the table.' Newlands arranged all of the known elements, starting with hydrogen and ending with thorium, into seven groups of eight, which he likened to octaves of music.[3][4] In Newlands' table, the elements were ordered by the atomic weights that were known at the time and were numbered sequentially to show their order. Periods were shown going down the table, with groups going across – the opposite from the modern form of the periodic table.
The incompleteness of the table alluded to the possible existence of additional, undiscovered elements, such as the element germanium, which was predicted by Newlands.
The Law of Octaves was ridiculed by Newlands' contemporaries, and the Society of Chemists did not accept his work for publication.[5]
Jump to Next: Uncle Tungsten - XI. Spectroscopy
No comments:
Post a Comment